BioImaging Facility
- The facility has reopened. Masks are no longer required
- Reserving equipment at http://bookit.hunter.cuny.edu prior to use is mandatory
 |
 |
|
Managing Director |
Scientific Director |
 |
 |
Description of the Facility
Background Overview
The BioImaging Facility at Hunter College is centered in a multi-room facility of 1024 sq. ft. located in the Biological Sciences Department on the 8th Floor of Hunter North building. A satellite facility also includes a number of instruments on the 4th Floor of the Belfer Research building (at 69th Street and York Ave). Faculty and students have access to a broad spectrum of instruments, ranging from simple white light wide-field microscopes to fluorescent multidimensional super-resolution and confocal imaging systems. The Faculty supervisor and Scientific Director is Dr. Diana P. Bratu. Dr. Lloyd Williams is the Managing Director of the facility. The facility staff has expertise in many areas of microscopy including the laser scanning confocal microscopy, super-resolution microscopy, two-photon microscopy. They are also familiar with many image analysis software packages, including, Imaris, Volocity, Autoquant, MetaMorph, and NIS-Elements. Detailed descriptions of the equipment in the facility is given below. All equipment is located at Rm 826 HN or at the 4th floor of the Belfer Research Building where designated
To book time on any of the instrumets go to http://bookit.hunter.cuny.edu
Instruments
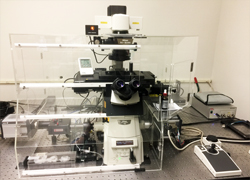 |
Nikon Eclipse Ti, TIRF/SIM
This machine is in 826HN |
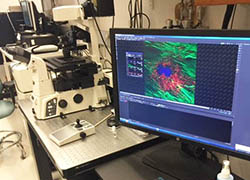 |
Belfer Nikon A1 Confocal Microscope The Nikon A1 Confocal microscope is Nikon's powerful fully-automated confocal imaging system, capable of capturing high-quality confocal images of cells and molecular events at high speed and enhanced sensitivity. The acquisition software is NIS-Elements. The system is located at Belfer Research Building. The charge for this instrument is $20/hr. |
 |
Olympus VS200 Slide Scanner The SLIDEVIEW™ VS200 research slide scanner enables you to capture high-resolution images of slides for quantitative analysis. The charge for this instrument is $20/hr. |
 |
Nikon Eclipse Ti Mosaic System
This machine is in 826HN |
 |
Perkin Elmer UltraView ERS This machine is in 830HN The charge for this machine is $20/hr |
 |
Leica Confocal TCS SP8 DLS
This machine is in 830HN |
 |
Leica Confocal Microscope TCS SP2
This machine is in 826HN |
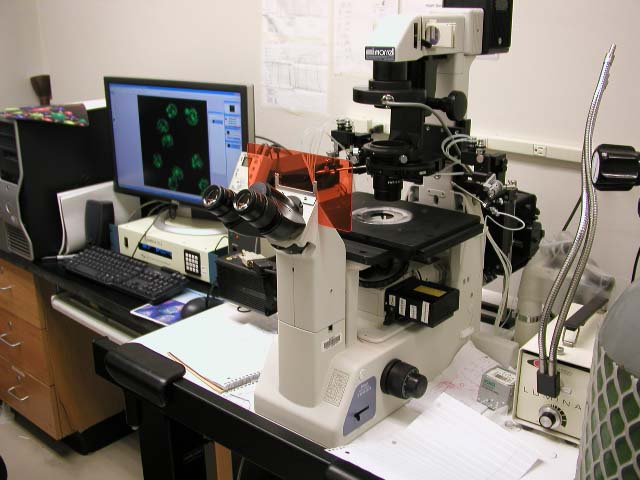 |
Nikon Eclipse TE 200 Calcium Ratio & Micro Injection
This machine is in 826HN |
 |
Belfer Nikon Ti-S Fluorescence Microscope The Nikon Ti-S microscope has a SOLA Light Engine solid state light source and a Nikon DigiSight camera. It has filter sets for DAPI FITC and RFP The charge for this instrument is $5/hr. |
 |
JEOL JEM-100C/CX Transmission Electron Microscope JEOL JEM-100CX II transmission electron microscope is an advanced high-performance electron microscope with stable and excellent imaging capabilities at low to high magnifications (90X to 800, 000X). A 10M-pixel HAMAMATSU C4742-95 digital camera is integrated into the system for high-resolution image acquisition. |
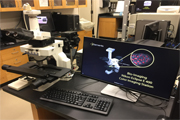 |
Nikon Eclipse E 400 Color Image Analysis System The Nikon Color Imaging system consists of a Nikon Eclipse E400 upright microscope, and Nikon DXM 1200F high-resolution (3,840 x 3,072 pixels) color video camera. The microscope is equipped for fluorescence and has phase contrast and Nomarski optics. The system is interfaced to a 1.4Ghz PC which utilizes Nikon Imaging Software. The system also has Adobe Photoshop installed for image acquisition and manipulation The charge for this instrument is $5/hr. |
 |
Imaris 8.41 Imaging Station The Imaris Imaging station is a high-power workstation with Bitplane's Imaris Imaging software installed. Imaris provides functionality for the visualization, segmentation, and interpretation of 3D and 4D microscopy datasets. the charge for this instrument is $10/hr. |
 |
Imaris 9.12 Imaging Station This Imaging workstation is a high-power workstation with Nikon's NIS-Elements Imaging software installed. NIS-Elements provides cutting edges tools for image manipulation and data management. It also has Imaris 9.12 installed The charge for these instruments is $5/hr for Elements and $10 per hour for Imaris. |
 |
Autoquant Deconvolution Station
This machine also has a floating license of Imaris 9.6 |
 |
Belfer NIS-Elements Analysis with Deconvolution This Imaging workstation has Nikon's NIS-Elements Imaging software installed. Additionally, it has Element's deconvolution module installed. This machine also has a floating license of Imaris 9.6 The charge for this instruments is $5/hr for Elements and and $10 for Imaris. |
 |
Gemini EM Microplate Spectrofluorometer The Molecular Devices SpectraMax Gemini EM Microplate Spectrofluorometer features top and bottom reading optics, dual monochromators, wavelength scanning, well scanning, auto PMT gain and is driven by Softmax Pro software on a Windows-based controller. The charge for this instrument is $5/scan. |
 |
Amersham Biosciences Typhoon 9410 Typhoon is a highly sensitive variable-mode gel imager. The Typhoon 9410 unites the ability to detect an extensive variety of fluors, with proven storage phosphor autoradiography technology and direct imaging of chemiluminescence. The typhoon can also be used to analyze microarrays. The charge for this instrument is $5/scan. |
 |
Belfer GE FLA 7000 Typhoon Typhoon FLA 7000 is a fast laser scanner for biomolecular imaging applications including sensitive and quantitative measurements of radioisotopic labels, chemifluorescent Western blots, and single fluorescence. The charge for this instrument is $5/scan. |
 |
Odyssey Infrared Imager The Odyssey replaces the tradition methods of analyzing western blots, chemiluminescence, and fluorescence detection, with near-infrared (NIR) fluorescence detection. This allows you to quantitate proteins over a much wider linear dynamic range than chemiluminescence can. This feature can enable strong and weak bands on the same blot to be accurately quantified without the need for multiple exposures. In addition, the Odyssey is uniquely equipped with two infrared channels 700 nm and 800 nm, and can thus probe two different targets in the same experiment. The charge for this instrument is $5/scan. |
 |
Biotek PowerWave Microplate Reader PowerWave HT is a multi-channel reader for maximum speed in both 96- and 384-well plate formats. The PowerWave HT provides flexibility for multiple applications, including endpoint, kinetic and spectral scanning mode. Powerful Gen5 PC-based software is used for system control and data analysis. The charge for this instrument is $3/scan. |
 |
Belfer Bio Tek Synergy HTX Microplate Reader
This instrument is in room BB 453 |
 |
GloMax®-96 Microplate Luminometer The GloMax®-96 Microplate Luminometer is a state-of-the-art Microplate Luminometer with a high sensitivity and broad dynamic range that is necessary for chemiluminescent and bioluminescent applications. The GloMax uses an advanced photon-counting photomultiplier tube (PMT), with a dynamic range of greater than nine logs. This range covers virtually all chemiluminescent and bioluminescent assays, eliminating the need to dilute samples or manage detector-driven gain changes. The charge for this instrument is $5/scan. |
 |
Leica CM 3050S Cryostat The Leica CM 3050S Cryostat features motorized sectioning and programmable defrost cycles. The cryostat can cut sections in the range 0.5 to 300 mm. The max specimen size is 55 X 70 mm and can cool samples down to - 50°C. The charge for this instrument is $5/hr. Sign the log book when you use this system To book time on this system use the Cryostat SharePoint Calendar at |
 |
Imaris For Core Facilities The facility has a floating Imaris license that can be installed on certain machines on the 8th and 9th floor of Hunter North. The charge for using this license is $10 per hour. We also have satellite license that can be issued for off-campus use. The charge for this is $10 for a day or $40 for a week |
Rules of Operations
Guidelines For using The Facility
A. The facility is open for use by members of the CTBR, other CUNY departments, and outside parties with the prior arrangement of the staff of the Bio-Imaging facility
B. Your use of the facility will be recorded. For the optical microscopes and the Gel and Blot scanners, you must obtain a "Gene Center" computer account. This is required to log on to the computers that control the equipment. Your use of the machine will then be automatically logged and you will be charged according to the fee schedule below.
C. The facility is available for use 7 X 24. After normal working hours ( 9-5 Mon-Fri ) lock the door when you leave, and access using a key obtained from the facility.
D. Training is required before using all machines. This can be done by individually with the facility manager, or by experienced users in the various CTBR laboratories. For the three confocals, and the Nikon SIM/TIRF you must be trained by the facility managers. Once you have complete the training course your account will be activated for the microscope.
E. When using the Cryostat, sign the logbook.
F. If you encounter problems with the facility E-mail the facility director Lloyd Williams at
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
.
G. Do not wear latex gloves in the facility
H. Do not leave your samples in the facility
I. Turn off all microscope lamps after use. It is particularly important to turn off the mercury lamps. Once mercury lamps have been turned off do not turn them back on until they have cooled down for 15 minutes
J. Report mercury lamps in service for more than 300 hours
K. Equipment is available on a first come first serve basis. You can book 3 hours slots on the microscopes using the facility reservations website: http://bookit.hunter.cuny.edu. If you come 30 minutes late you will lose your reservation. You can create an account to make reservations on the site. You will need a "Gene Center" network account to use most instuments.
L. Users may have no more than 2 reservations made on a calendar at one time for any single machine.
For example, Monday 12-4pm, Tues 9-1pm. After Monday's session, the user may schedule another session, thus obtaining another/2nd appointment on the calendar, say Wed 2-5pm.
M. Clean oil off the microscope objective lenses after use.
N. Report all accidents (injuries, spills, fires) to the Security (x4444) and Health and Safety Officer, Ricardo Franco (x4462)
O. You must log in to use the equipment using your own Gene Center account.
P. Publications using data taken in this facility must acknowledge the RCMI program and the NCRR. Suggested language for the acknowledgment would be,
"This project was supported by a Research Centers in Minority Institutions Program grant from the National Institute on Minority Health and Health Disparities (MD007599) of the National Institutes of Health."
Fee Schedule
PerkinElmer Spinning Disk Microscope:
The facility charges $20 per hour for use of this microscope. There is a $20 minimum charge, and fractions of an hour count as whole hours. For long time duration experiment, we have a special rate policy described as follows: in any 24 hour period
0 - 4 hours $20/hour
4 - 10 hours $10/hour
10 - 24 hours $5/hour
Email Lloyd Williams (
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
) in advance for applying this policy.
Leica Sp2 Confocal: The facility charges $20 per hour for use of the confocal. There is a $20 minimum charge, and fractions of an hour count as whole hours.
Leica Sp8 Confocal: The facility charges $20 per hour for use of the confocal. There is a $20 minimum charge, and fractions of an hour count as whole hours.
Nikon SIM/TIRF: The facility charges $20 per hour for use of this microscope. There is a $20 minimum charge, and fractions of an hour count as whole hours.
Nikon Eclipse Ti With Ultra High-Speed Wavelength Source: The facility charges $15 per hour for use of this microscope. There is a $15 minimum charge, and fractions of an hour count as whole hours.
All Other Nikon Upright & Inverted Microscopes: The facility charges $10 per hour for use of the microscope. There is a $10 minimum charge, and fractions of an hour count as whole hours.
Leica CM 3050S Cryostat: The facility charges $5 per hour for use of the Cryostat. There is a $5 minimum charge, and fractions of an hour count as whole hours. Please sign the log book.
Gemini Spectrophotometer, Typhoon 9410 Imager: The facility charges $5 per scan. Use is monitored by the event log on the computer attached to the machines.
PowerWave HT Plate Reader: The facility charges $3 per scan. Use is monitored by the event log on the computer attached to the machines.
Elements Analysis Workstation: The facility charges $5 per hour for use of this image analysis package. There is a $5 minimum charge, and fractions of an hour count as whole hours.
Volocity Analysis Workstation: The facility charges $10 per hour for use of this image analysis package. There is a $10 minimum charge, and fractions of an hour count as whole hours.
Imaris Analysis Workstation: The facility charges $10 per hour for use of this image analysis package. There is a $10 minimum charge, and fractions of an hour count as whole hours. The charge for use of the core facilities version of Imaris is also $10 /hour. Satellite license use is $10 per day or $40 per week.
Remote Instrumentation
Purpose
Nowadays, microscopic imaging techniques are becoming more and more popular in biomedical research. Most of the advanced high-end microscope systems (i.e., confocal & spinning disk systems) are expensive, not every research lab can have enough fund to support such system for their research. A solution to overcome this problem is to share the microscope systems through the Internet (also called remote instrumentation): remote users can get the control of the microscope for their experiment through a simple Internet connection. Our approach for this remote instrumentation task is to combine the powers of WebEx and PVX:
(i) Utilize WebEx to setup remote desktop sharing for microscope control.
(ii) Utilize PVX monitoring system to setup Internet video conferencing for remote communication purpose.
Remote instrumentation service
Now a new service is ready for our remote users to get remote access to our advanced confocal microscopes for their own imaging purpose.
Our service includes:
(i) Leica SP2 confocal microscope: it is ideal for regular 2D & 3D scanning for fixed slide samples. Please check the following link for Leica SP2 system: Leica SP2 operational guide;
(ii) Perkin Elmer spinning disk microscope: besides the regular 2D & 3D fixed slide scanning, it is also equipped with an environment chamber for live cell imaging. Also, this type of microscope system has fast scanning speed, it is ideal for cellular dynamic studies. Please check the following link for Perkin Elmer spinning disk microscope system: Spinning disk operational guide;
(iii) Microscope remote control: Webex is used to setup the remote desktop sharing for microscope control. Please check the following link for WebEx-based remote control operations: Webex remote control guide;
(iv) PVX video conferencing for real-time consultation: during imaging experiment, PVX video conferencing system is used for real-time conversations between microscope operator and remote users to solve on-site experimental issues. Please check the following link for PVX real-time conferencing: PVX video conferencing operational guide.
(v) Cell staining protocol: a simple cell staining protocol is posted here as an example: Sample staining protocol.
For scheduling the above remote instrumentation service, please check the following guidelines:
-
Contact us by email for scheduling a remote microscopic imaging experiment, including, a short description of your experiment, time schedule, sample type, etc.
Contact emails:- Dr. Lloyd Williams: This e-mail address is being protected from spambots. You need JavaScript enabled to view it
- Ship the sample slide or living samples with proper package.
- A confirmation email will be sent before the experiment date. A WebEx meeting link will be included in this email for remote connection.
Application Summary for Different Readers in Bio-Imaging Facility
|
Glomax 96 Microplate Luminometer |
Biotek PowerWave HT Plate Reader |
SpectraMax Gemini EM Fluorescence Spectrometer |
Typhoon 9410 Imager | LI-COR Odyssey | |
|---|---|---|---|---|---|
| Sample Type | 96-well plate | 96 & 384-well plate | 96 to 384-well plate | Plate/Gel/Blot | Western blot sample |
|
Excitation Wavelength Range |
N/A | 200-999nm | 250-850nm |
457nm/488nm/ 532nm/633nm |
680nm/780nm |
|
Detection Wavelength Range |
300-650nm | 200-999nm | 360-850nm | 500-685nm | Near-infrared detection 680-1000nm |
| Detection Mode | Luminescence | Absorbance | Fluorescence | Phosphorimaging Chemiluminescence Fluorescence | Fluorescence |
| Read Mode | Endpoint/Kinetics | Endpoint/Kinetics |
Endpoint/Kinetics /Spectrum |
Image | Image |
| Application | Chemiluminescence Bioluminescent assay |
Direct DNA quantitation Purity testing RNA quantitation ELISA Enzyme Kinetics Colorimetric assays |
ELISAs and Immunoassays Nucleic Acid Quantitation Protein Quantitation Reporter Gene Assays Cell Viability, Proliferation, and Cytotoxicity Enzyme Assays Transporter Assays Phosphatases/Kinases Microbial Growth |
Quantitative Phosphorimaging ECL Plus Westerns Multifluorescence applications (such as 2-D DIGE and ECL Plex) |
Quantitative Western ELISA/FLISA In-cell Western Assay In-Gel Western Assay On-cell Western Assay Microwestern |
Objectives of Microscopes in the Bio-imaging Facility
| Lens\System | Nikon Upright | Nikon Inverted | Leica Confocal | PE Spinning-disk | Nikon TIRF/SIM | Nikon A1 | Leica SP8 |
|
Objective Description Magnification/NA /Immersion (dry lens unless mentioned) |
4x/0.13 | 4x/0.1 | 5x/0.15 | 20x/0.45/multi | 40x/0.6 | 100x/1.45/oil | 100x/1.40/oil HC PL APO |
| 10x/0.45 | 10x/0.3 | 10x/0.4 | 60x/1.49/oil | 60x/1.49/oil | 60x/1.45/oil | 63x/1.40/oil HC PL APO | |
| 20x/0.75 | 20x/0.45 | 20x/0.5 | 100x/1.4/oil | 100x/1.49/oil | 40x/1.25/oil | 40x/1.3/oil HC PL APO | |
| 40x/1.0/oil | 40x/0.6 | 40x/1.25/oil | 40x/1.3/oil | 20x/0.75 | 10x/0.40 HC PL APO | ||
| 60x/1.4/oil | 63x/1.4/oil | 10x/0.45 | 5x/0.15 HC PL FLUOTAR | ||||
| 100x/1.4/oil | 2.5x/0.007 HC PL FLUOTAR |
The Leica SP8 also has the following DLS objectives 1.6x/0.05, 5x/0.15, 10x/0.3, 25x/0.95
and DLS TwinFlect 2.5mm, 5mm, 7.8 mm(water) 7.8mm (glycerin)
Laser wavelength specifications for various instruments
|
Leica SP2 Confocal, Room 826 HN |
|
|
Argon Ion Laser |
458, 476, 488, 514 nm |
|
Melles Griot Solid State Laser |
561 nm |
|
HeNe Laser |
633 nm |
|
PE Spinning-disk Room 826 HN |
|
|
Solid State Laser |
561 nm |
|
Solid State Laser |
405 nm |
|
Solid State Laser |
488 nm |
|
Solid State Laser |
514 nm |
|
Solid State Laser |
440 nm |
|
Solid State Laser |
640 nm |
|
Nikon TIRF/SIM Room 826 HN |
|
|
TIRF Module |
|
|
Solid State Laser |
405 nm |
|
Solid State Laser |
488 nm |
|
Solid State Laser |
561 nm |
|
Solid State Laser |
640 nm |
|
SIM module |
|
|
Solid State Laser |
488 nm |
|
Solid State Laser |
561 nm |
|
Solid State Laser |
640 nm |
|
Nikon Eclipse Ti Mosaic/MicroPoint System & FRAP Room 826 HN |
|
|
Solid State Laser |
405 nm |
|
Amersham Biosciences Typhoon 9410 Room 826 HN |
|
|
Argon Ion Laser |
457, 488 nm |
|
SYAG laser |
561 nm |
|
HeNe Laser |
640 nm |
|
Nikon A1R Resonant Confocal ( Room Belfer BB 479). |
|
|
Solid State Laser |
405 nm |
|
Solid State Laser |
488 nm |
|
Solid State Laser |
561 nm |
|
Solid State Laser |
640 nm |
|
GE FLA 7000 Typhoon (Room Belfer BB ) |
|
|
Solid State Laser |
473 nm |
|
Solid State Laser |
532 nm |
|
Solid State Laser |
635 nm |
|
Solid State Laser |
650 nm |
Website:
Biology Department



